Block (periodic table)
a set of elements in the periodic table of elements, defined by shape of an orbital—s, p, d, or f—where the valence electron lies
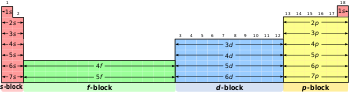
A block on the periodic table is a group of elements that all have their electrons in the same atomic orbital. There are four blocks, s-, d-, f, and p-.[1] The word "block" was first used to describe this by Charles Janet.[2]
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Period | |||||||||||||||||||
| 1 | 1 H |
2 He | |||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | |||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | |||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | |
| 6 | 55 Cs |
56 Ba |
57 La |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn | |
| 7 | 87 Fr |
88 Ra |
89 Ac |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og | |
| 8 | 119 Uue |
120 Ubn |
121 Ubu | ||||||||||||||||
| 58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu | ||||||
| 90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr | ||||||
| 122 Ubb |
123 Ubt |
124 Ubq |
125 Ubp |
126 Ubh |
127 Ubs | ||||||||||||||
Blocks
changep-block
changeThe p-block is on the right side of the periodic table. Elements from groups 13-18 are in the p-block.
d-block
changeThe d-block is in the middle of the periodic table. Elements from groups 3 to 12 are in the d-block.
f-block
changeThe f-block is shown in green in the illustration above. The elements 57 to 71 are called Lanthanides, elements 89 to 103 are called Actinides.
s-block
changeThe s-block is on the left side of the periodic table. Elements from groups 1-2 are in the s-block.
References
change- ↑ 1.0 1.1 Jensen, William B. (21 March 2015). "The positions of lanthanum (actinium) and lutetium (lawrencium) in the periodic table: an update". Foundations of Chemistry. 17: 23–31. doi:10.1007/s10698-015-9216-1. S2CID 98624395.
- ↑ Charles Janet, La classification hélicoïdale des éléments chimiques, Beauvais, 1928