User:Mr. Ibrahem/Ciprofloxacin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ciloxan, Cipro, Neofloxin, others |
| AHFS/Drugs.com | Systemic: Monograph Eye and ear: Monograph |
| MedlinePlus | a688016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical (ear drops, eye drops) |
| Drug class | Fluoroquinolone |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70%[2] |
| Protein binding | 30%[2] |
| Metabolism | Liver (incl. CYP1A2) |
| Elimination half-life | 3.5 hours[2] |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
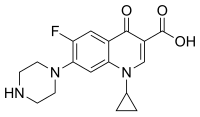
| Formula | C17H18FN3O3 |
| Molar mass | 331.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ciprofloxacin is an antibiotic used to treat a number of bacterial infections.[3] This includes bone and joint infections, intra abdominal infections, certain type of infectious diarrhea, respiratory tract infections, skin infections, typhoid fever, and urinary tract infections, among others.[3] For some infections it is used in addition to other antibiotics.[3] It can be taken by mouth, as eye drops, as ear drops, or intravenously.[3][4]
Common side effects include nausea, vomiting, diarrhea and rash.[3] Severe side effects include an increased risk of tendon rupture, hallucinations, and nerve damage.[3] In people with myasthenia gravis, there is worsening muscle weakness.[3] Rates of side effects appear to be higher than some groups of antibiotics such as cephalosporins but lower than others such as clindamycin.[5] Studies in other animals raise concerns regarding use in pregnancy.[6] No problems were identified, however, in the children of a small number of women who took the medication.[6] It appears to be safe during breastfeeding.[3] It is a second-generation fluoroquinolone with a broad spectrum of activity that usually results in the death of the bacteria.[3][7][8]
Ciprofloxacin was patented in 1980 and introduced in 1987.[9][10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic medication.[3][12] The wholesale cost in the developing world is between US$0.03 and US$0.13 a dose.[13] In the United States it is sold for about US$0.40 per dose.[3] In 2017, it was the 107th most commonly prescribed medication in the United States, with more than six million prescriptions.[14][15]
References
change- ↑ 1.0 1.1 "Ciprofloxacin Use During Pregnancy". Drugs.com. 7 January 2019. Archived from the original on 27 October 2019. Retrieved 19 December 2019.
- ↑ 2.0 2.1 2.2 Zhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, Gin AS, Rubinstein E, Hoban DJ (2006). "A Review of New Fluoroquinolones : Focus on their Use in Respiratory Tract Infections". Treatments in Respiratory Medicine. 5 (6): 437–65. doi:10.2165/00151829-200605060-00009. PMID 17154673.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 "Ciprofloxacin Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 23 September 2015. Retrieved 23 August 2015.
- ↑ "Ciprofloxacin Hcl Drops". WebMD. 22 Feb 2018. Archived from the original on 23 February 2018. Retrieved 22 Feb 2018.
- ↑ Heidelbaugh JJ, Holmstrom H (April 2013). "The perils of prescribing fluoroquinolones". The Journal of Family Practice. 62 (4): 191–7. PMID 23570031. Archived from the original on 28 August 2021. Retrieved 30 July 2020.
- ↑ 6.0 6.1 "Prescribing medicines in pregnancy database". Australian Government. 23 August 2015. Archived from the original on 8 April 2014.
- ↑ Ball P (July 2000). "Quinolone generations: natural history or natural selection?". The Journal of Antimicrobial Chemotherapy. 46 Suppl T1: 17–24. doi:10.1093/oxfordjournals.jac.a020889. PMID 10997595.
- ↑ Oliphant CM, Green GM (February 2002). "Quinolones: a comprehensive review". American Family Physician. 65 (3): 455–64. PMID 11858629. Archived from the original on 28 August 2021. Retrieved 30 July 2020.
- ↑ Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009. p. 56. ISBN 978-0-19-103962-1. Archived from the original on 8 September 2017.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 500. ISBN 9783527607495. Archived from the original on 29 December 2016. Retrieved 30 July 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Hamilton, Richard J. (2014). Tarascon pharmacopoeia (15 ed.). Jones & Bartlett Publishers. p. 85. ISBN 978-1-284-05671-6. Archived from the original on 8 September 2017.
- ↑ "Ciprofloxacin". International Drug Price Indicator Guide. Archived from the original on 3 April 2019. Retrieved 24 August 2015.
- ↑ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ "Ciprofloxacin - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.