Crystal structure
In crystallography, the crystal structure is how the atoms (or ions or molecules) are arranged in a crystalline material.[3] Crystals occur naturally from the way the chemical bonds of the atoms connect. Symmetric repeating patterns occur in 3-D space in the crystal.
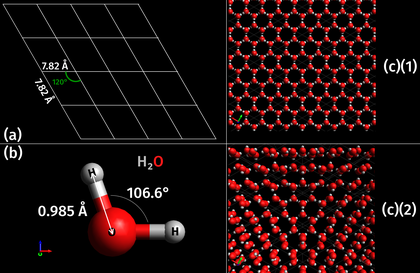
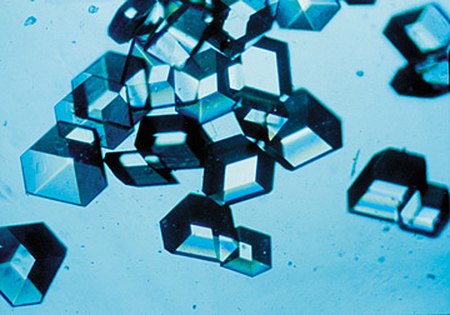
The crystal structure and symmetry cause many physical properties, such as cleavage (how the crystal splits) how it conducts electricity, and its optical properties..
The crystal structure of a chemical is the shape of the crystal at the molecular level. There are several shapes of crystals. Sodium chloride is cubic. Copper sulfate is triclinic. Most things, even metals, have crystal structures. Some crystals fit more atoms in them than others, and these crystals usually weigh more.
Each type of crystal structure has a unit cell, which is a small pattern of points that repeats through the whole crystal. For example, in a primitive cubic crystal, the unit cell is the eight corners of a cube. When many unit cells are next to each other, you get a crystal.
References
change- ↑ Petrenko, V. F.; Whitworth, R. W. (1999). Physics of Ice. Oxford University Press. ISBN 9780198518945.
- ↑ Bernal, J. D.; Fowler, R. H. (1933). "A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions". The Journal of Chemical Physics. 1 (8): 515. Bibcode:1933JChPh...1..515B. doi:10.1063/1.1749327.
- ↑ Hook, J.R.; Hall, H.E. (2010). Solid State Physics. Manchester Physics Series (2nd ed.). John Wiley & Sons. ISBN 9780471928041.